Australian Pulse Bulletin
Chickpea: Ascochyta blight management
Kevin Moore1, Mal Ryley2, Gordon Cumming3, Leigh Jenkins1
Photos: Gordon Cumming
1 NSW Department Primary Industries, 2 Queensland Department of Agriculture and Fisheries, 3 Pulse Australia
Background
Ascochyta blight, caused by the fungus Phoma rabiei (formerly Ascochyta rabiei), is a serious disease of chickpeas in Australia. The fungus can infect all above ground parts of the plant and is most prevalent when cool, cloudy and humid weather occurs during the crop season.
Ascochyta blight first caused widespread damage to chickpeas in northern New South Wales and southern Queensland in 1998 when extremely wet conditions favoured disease development and spread. Ascochyta blight is now considered to be endemic in all growing regions with the exception of central Queensland. Unlike some insect control strategies, there is no economic threshold for ascochyta. Management strategies are aimed at preventing the occurrence of disease and limiting its spread.
Ascochyta blight is managed through crop rotation, hygiene, seed treatment, prophylactic fungicide application and growing varieties with improved resistance (Table 1).
All growers and advisors need to inspect their crops regularly, from emergence through flowering right up to plant maturity. Inspections should be undertaken 10–14 days after rain events, when new infections will be clearly evident as lesions on plant parts.
Initial crop infection is due to the introduction of either infected planting seed or from movement of infected trash by wind, machinery or animals. Spores of the fungus can survive for a short time on skin, clothing and machinery. Subsequent in-crop infection and spread occurs when inoculum is moved higher in the canopy or to surrounding plants by wind or rain splash during wet weather. There are no other known hosts of Phoma rabiei in Australia.
Identification and visual symptoms
-

Usually the initial symptoms are the wilting of individual or small groups of seedlings.
-

Plants appear as if premature haying off has occurred.
-

Lesions usually begin as a pale green-yellow discolouration on leaves and stems and progress into small round lesions with dark-brown margins and pale grey to tan sunken centres.
-

Note the concentric circles of brown–black dots in the centre of the lesions. These pycnidia or fruiting bodies are unique to ascochyta blight.
-

Pycnidia produce spores, which infect other plants through wind-borne stubble and/or water (rain-splash).
-

Ascochyta leaf ghosting symptoms may appear 4–7 days after rainfall or heavy dew.
-

Lesions on stems at first tend to be oval shaped, with brown centres and a darker margin.
-

Pycnidia are also present in stem lesions.
-

Stem lesions can cause girdling and breakage.
-

Ascochyta infected stems and leaves.
-

Pod lesions are similar in appearance to leaf lesion. They lead to infection of the seed.
Phoma rabiei infects the leaves, stems and pods of chickpea plants, causing tan/brown, rounded lesions on affected plant parts. Symptoms become visible in 4–5 days as a pale green/yellow discolouration on leaves, often referred to as ‘ghosting’ (see image). Toward the centre of the lesion, small, black fruiting bodies called pycnidia develop in 7–10 days, often in concentric rings (see image). Spores ooze out of pycnidia and are spread by rain-splash upwards within the plant and sideways to nearby healthy plants. Lesions often girdle the stems of the plant, causing them to weaken and subsequently break off, making later detection difficult. Circular 'hot spots' or 'foci' consisting of plants with severe infection can appear in crops but by this stage considerable damage has occurred. Seeds can become infected after lesions develop on pods.
Initial symptoms are commonly the wilting of individual or small groups of seedlings. Patches of wilted, pruned, and dying plants develop within the crop; these patches appear as premature haying off. Circular spots with tan to grey centres with dark brown to black margins develop on leaves, stems and pods.
- Leaf lesions: Lesions usually begin as a pale green-yellow discolouration on leaves and stems and progress into small round lesions with dark-brown margins and pale grey to tan sunken centres. Toward the centre of the lesion, fruiting bodies called pycnidia develop (appearing as black specks), often in concentric rings. These pycnidia produce spores, which spread on wind-borne stubble and/or water (rain-splash) to infect other plants. Note the concentric circles of brown-black dots in the centre of the lesions. These are the pycnidia or fruiting bodies that are unique to ascochyta blight. Ascochyta leaf ghosting may appear 4–7 days after infection following rainfall or heavy dew.
- Stem lesions: Lesions on stems at first tend to be oval shaped, with brown centres and a darker margin. Lesions often girdle the stems of the plant, causing them to weaken and subsequently break off.
- Pod lesions: Pod lesions are similar in appearance to leaf lesions. They lead to infection of the seed. DO NOT keep planting seed from any crop that has been identified as having ascochyta blight.
Visual effects that are NOT ascochyta blight:
- Frost is the most common cause of leaf necrosis (whitening, death) which might be confused with Ascochyta blight. Herbicide injury (eg: simazine, Balance®) may also cause similar effects.
- However, there are clearly no pycnidia (fruiting bodies) present.
- Other causes of branch death can include frost, and physical damage from wind, mice, other animals as well as the passage of machinery.
-

Note the absence of pycnidia (fruiting bodies).
-

Consider other causes where pycnidia are not present on damaged leaves.
-

Non-disease causes of branch death can include physical damage from wind, mice, other animals and the passage of machinery.
-

Frost is the most common non-ascochyta cause of the ghosting effect on leaves.
-

Some herbicide (e.g simazine, Balance®) injury can cause the ghosting effect.
Biology and epidemiology
Phoma rabiei causes economic losses only on chickpea. There are no other known hosts of the pathogen in Australia, but different Ascochyta species infect faba beans, lentils and field peas. P. rabiei survives between seasons on infected plant residues, on infected or contaminated seeds and on infected volunteer chickpea plants.
P. rabiei infected stubble blown about during and after harvest is a major cause of short to medium distance dispersal (metres to kilometres) along with movement of infected trash by water, machinery or animals. Spores of the fungus can survive a short time on skin, clothing and machinery.
P. rabiei can increase rapidly on volunteer chickpeas, if wet weather occurs during spring-summer-autumn. Paddocks with chickpea stubble should be regarded as a source of inoculum even if ascochyta blight was not observed in last season’s chickpea crop.
P. rabiei can develop over a wide range of temperatures (5–30°C) and needs only 3 hrs of leaf wetness to infect. However, the disease develops fastest when temperatures are between 15–25°C and relative humidity is high (the longer the Relative Humidity is high, the more sever will be the infection).
Subsequent in-crop infection occurs when spores are moved higher in the canopy or to surrounding plants by rain splash during wet weather. Multiple cycles of infection will occur during the growing season whenever environmental conditions are favourable.
Crop inspection and hygiene
- When inspecting crops, look for signs of wilting in upper foliage and small areas of dead or dying plants.
- Check in a range of locations across the field following a ‘V’ or ‘W’ pattern.
- Spend at least 1 to 2 hour inspecting each crop for ascochyta blight.
- Ensure good hygiene when moving between crops and farms (see below).
Take extra care when inspecting crops that are growing:
- under centre pivot or lateral-move irrigators
- from seed whose ascochyta status is unknown
- from seed that was not treated with a registered fungicide seed dressing.
If ascochyta is suspected
If ascochyta is suspected mark the spot and take samples for diagnosis. DO NOT enter other chickpea paddocks wearing the same clothing. All other chickpea crops on the property need to be inspected for ascochyta blight. Be sure to follow the hygiene practices outlined below.
- Place samples of suspected ascochyta-infected plants into a plastic bag then seal the bag and keep the samples cool.
- Suspect samples should be referred to a plant pathologist or agronomist familiar with the disease for identification.
- Unnecessary movement within a suspected ascochyta-infected crop should be avoided until the sample has been fully assessed.
- Most importantly, do not visit other chickpea crops until all clothing has been disinfected or changed and machinery has been washed of all plant material and dirt.
Hygiene
The spores of ascochyta can adhere to clothing, machinery, vehicles, people and animals when moving through infected paddocks, so hygiene is a vital component of IDM when ascochyta is found in a crop.Wear waterproof pants, overboots or rubber gum boots when entering a suspected infected paddock, then decontaminate immediately after exiting.
- Farmers and advisors should take precautions to prevent spreading ascochyta blight via clothing, footwear and vehicles.
- The recommended protocol is for clothing to be washed, changed or disinfected when moving between chickpea paddocks.
- Wash boots in a mixture of 10% bleach and 90% water solution or methylated spirits upon leaving an infected chickpea crop.
- Clothing must be machine-washed in hot water before being worn when entering another chickpea crop.
- Extra care should be taken to remove soil and plant material from boots and vehicles.
- Hands and arms should be washed in warm soapy water or a suitable disinfectant.
- The use of heavy-duty plastic bags to cover boots and legs is a common practice when checking crops. After inspecting the crop, remove these plastic covers and place them in another bag and seal. Use another set of covers if you need to enter another chickpea crop.
- Farmcleanse® can be used to clean equipment.
During harvest
Harvest ascochyta-free paddocks before infected paddocks and preferably use your own harvester. Do not run the straw spreaders when harvesting, which will reduce the spread of small pieces of ascochyta-infected stem and pods.
Thoroughly clean and decontaminate all machinery associated with harvesting in a well-defined and identifiable area before moving to another paddock or property.
Post-harvest
All grain harvested from an ascochyta-infected paddock should be transported off-farm to receival sites in well sealed trucks. If kept for a period on-farm it should be stored in well-sealed and labelled silos which must be thoroughly cleaned after the grain has been removed.
Grain harvested from an ascochyta-infected crop must not be retained as planting seed for other crops.
Consideration may be given to incorporation of infected crop residues by the use of off-set discs immediately after harvest to enhance the rate of breakdown. Chickpea volunteers in the infected paddock, along fence lines and near sheds must be controlled.
Chickpeas should not be grown in or adjacent to an ascochyta-infected paddock for at least 3 years.
Determining your ascochyta blight risk
The northern GRDC region has three levels of ascochyta blight risk (low, medium and high), determined by:
- proximity to infection source
- seed infection and treatment
- variety grown
- likelihood of rain.
Ascochyta blight management strategies vary accordingly and are strongly influenced by weather conditions. The key to achieving cost effective management of ascochyta blight is to assess the risk level for each paddock, and then manage accordingly.
Chickpea growing areas of the northern region are classified as having a Low, Moderate or High ascochyta blight risk. Assessment should however be done on a paddock-by-paddock basis and not on a regional basis. For example, even though you might be in a western, drier area (which at the regional level carries a Moderate risk) you could have a high ascochyta risk if you have been growing chickpeas for many years, have sown a susceptible variety and are subject to seasonal conditions that favour the disease.
Low risk situations
The only region that currently can be considered Low Risk is central Queensland (Central Highlands and the Dawson Callide). Ascochyta blight was identified for the first time in central Queensland in spring 2008. If ascochyta becomes endemic in central Queensland, the industry will face considerable managerial and financial costs.
While the hot dry climate of central Queensland is less conducive to ascochyta than southern areas, the region is not immune, cool, wet winter conditions will favour the pathogen’s development and spread. Now that an incursion has occurred, growers in the region need to prioritise management of ascochyta to reduce the risk of the disease spreading within the region.
Primary (initial) infections result from infected seed or from infected crop residue. Seed must be sourced from within this region and treated with a registered fungicide seed dressing. Always check the origin of your seed prior to delivery and ensure that machinery, especially contract harvesters, entering the region has been thoroughly cleaned.
Chickpea varieties currently grown in central (& coastal) Queensland are all very susceptible to ascochyta blight.
Moderate risk situations
These occur when:
- chickpeas are sown at least 2 km away from inoculum sources, i.e. away from paddocks that had chickpeas or volunteers during the previous two seasons, and
- seed has come from crops where ascochyta was not detected and has been treated with a registered fungicide seed dressing.
Many crops in the western areas often meet these criteria i.e. west of the Darling Downs in Qld and west of the Newell highway in NSW. However, under favourable conditions and on susceptible varieties, ascochyta can build-up quickly and may reach epidemic levels.
High risk situations
Disease epidemics can develop rapidly in situations where chickpeas are grown within a 2 km radius of previous chickpea. Most of the more intensively farmed areas such as the Darling Downs and east of the Newell highway in NSW are in this High risk category. Paddocks tend to be smaller, making it difficult to stay 2 km away from infected chickpea crop residue and these areas are in higher rainfall zones. A number of crops in the western areas are potentially in this category, if they are sown within 2 km of paddocks that had chickpeas or volunteers during the previous two seasons.
Management options
Follow the principles of Integrated Disease Management (IDM), which include:
- crop rotation and paddock selection
- clean seed and fungicide seed dressings
- regular crop monitoring
- strict hygiene on and off farm
- strategic use of foliar fungicides
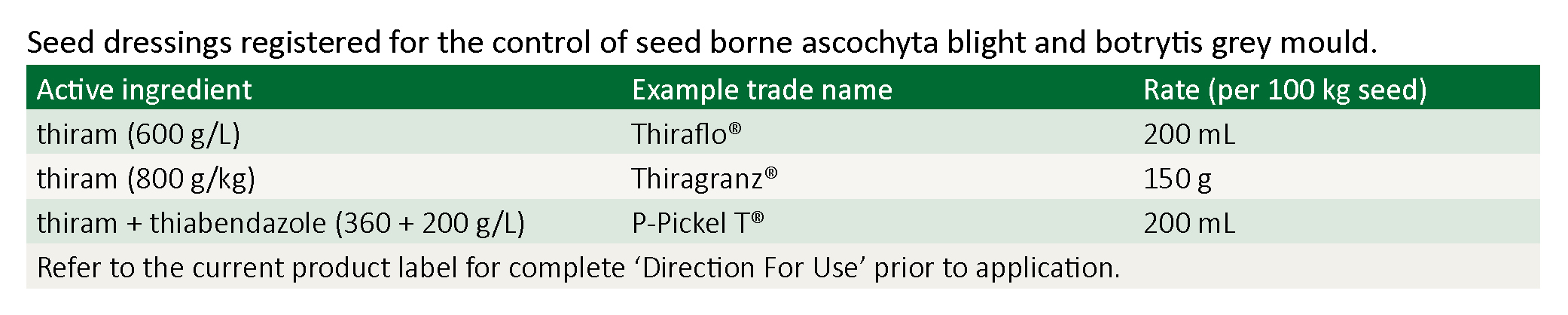
Note: Chickpea seed dressings only protect the emerging seedling from seed borne ascochyta and seed borne botrytis. Seed dressings will not protect the emerged seedling from rain-drop splashed ascochyta or wind borne botrytis.
For additional detailed information refer to: Pulse Australia - Northern Pulse Bulletin; ’Chickpea: Integrated Disease Management’
Foliar fungicide programs
Differing spray programs have been developed based on each variety’s ascochyta rating (see table below). Chickpea ascochyta fungicides are protectants only – unlike wheat stripe rust fungicides – they have no systemic or kick-back action, and they will not eradicate an existing infection. To be effective they must be applied before infection i.e. before rain. The key to a successful ascochyta spray program is regular monitoring combined with timely application of registered fungicides (see table below).
Note: Observations in 2010 Tamworth trials indicated that the natural resistance all plants have to pathogens and pests is compromised when plants are stressed from waterlogging and that this reduced the ability to manage ascochyta with a fungicide strategy that worked in less stressed plots. In a season when repeated cycles of infection occur, even MR varieties can have yield-reducing levels of disease.
Resistant (R) (e.g. GenesisTM 090, GenesisTM 425)
- Fungicide sprays are unlikely to be required before podding. Despite good foliar resistance to ascochyta, the flowers and pods of Resistant varieties can be infected, which can result in poor quality, discoloured seed or seed abortion and, in extreme situations, yield loss.
- Monitor the crop 10–14 days after each rain event.
- If ascochyta is detected, apply a registered fungicide at early podding prior to rain. In high rainfall or high risk situations and where there is an extended pod filling period, further applications may be required.
Moderately Resistant to Resistant (MR/R) (e.g. PBA HatTrick, PBA Boundary)
- In most seasons, disease development will be slow and there will be no or minimal yield loss. In such seasons there is no cost benefit in applying a fungicide during the vegetative stage. Despite good foliar resistance to ascochyta, the flowers and pods of MR/R rated varieties can be infected which can result in poor quality, discoloured seed or seed abortion and yield loss in severe situations.
- However, under high disease pressure, a reactive foliar fungicide strategy may be warranted during the vegetative period of the crop.
- Monitor the crop 10–14 days after each rain event.
- If ascochyta is present in the crop apply a registered fungicide at early podding prior to rain to ensure pods are protected, and high quality, disease free seed is produced.
Moderately Resistant (MR) (e.g. Flipper)
- In most seasons of low to moderate disease pressure, there is no cost benefit in applying a fungicide until after ascochyta blight is detected.
- Monitor the crop 10–14 days after each rain event and if ascochyta is detected apply a registered fungicide just before the next likely rain event.
- Continue monitoring and spray again if weather and disease levels indicate ascochyta is likely to spread.
Moderately Susceptible to Moderately Resistant (MS/MR) (e.g. Yorker, Almaz)
- For all situations apply a registered fungicide before the first rain event after crop emergence, or three weeks after emergence or at the three branch stage of development, whichever occurs first.
- Monitor the crop 10–14 days after each rain event.
- If ascochyta is found, apply a registered fungicide just before the next rain event.
- Continue monitoring and spray again if weather and disease levels indicate ascochyta blight is spreading.
Susceptible (S) varieties (e.g. Jimbour, Kyabra, Moti, PBA Pistol)
- If the season favours ascochyta, regular fungicide sprays will be needed from emergence until 4 weeks before maturity. Do not wait until you find the disease.
- Timing of the first two sprays is critical, because control is difficult or impossible after the disease has taken hold. The first spray must be applied before the first post emergent rain event, or three weeks after emergence or at the three leaf stage whichever occurs first. The second spray should be applied three weeks after the first spray. However, apply the second spray if two weeks have elapsed since the first spray and rain is forecast.
- Mancozeb is often the preferred fungicide for these first two applications as it can be applied with a Group A grass herbicide.
- Continue monitoring the crop 10-14 days after each rain event. If ascochyta is found additional sprays will be required. If it has been two weeks or longer since the last application, spray again just before the next rain event.


Useful resources
- Chickpea: Sourcing High Quality Seed
- Chickpea: Effective Crop Establishment
- Integrated disease management in chickpea
- Chickpea best management guide—Northern region
- Chickpea best management guide—Southern region
Key contacts
Disclaimer
Information provided in this guide was correct at the time of the date shown below. No responsibility is accepted by Pulse Australia for any commercial outcomes from the use of information contained in this guide.
The information herein has been obtained from sources considered reliable but its accuracy and completeness cannot be guaranteed. No liability or responsibility is accepted for any errors or for any negligence, omissions in the contents, default or lack of care for any loss or damage whatsoever that may arise from actions based on any material contained in this publication.
Readers who act on this information do so at their own risk.
Copyright © 2015 Pulse Australia
All rights reserved. The information provided in the publication may not be reproduced in part or in full, in any form whatsoever, without the prior written consent of Pulse Australia.
Last updated: 20 November 2015
