Australian Pulse Bulletin
Lentil: Integrated disease management
Wayne Hawthorne1, Michael Materne2, Jenny Davidson3, Kurt Lindbeck4, Larn McMurray1 and Jason Brand2
1Pulse Australia; 2Department of Primary Industries, Victoria; 3SA Research & Development Institute; 4Department of Primary Industries NSW.
Key points
- Paddock selection. Aim for a break of at least 3 years between lentil crops and at least 250 m from the previous year’s lentil paddock. Avoid sowing adjacent to faba bean, vetch, chickpea or lathyrus stubble.
- Variety selection. Select varieties with least susceptibility to the main disease risk for the region. Spread overall risks by sowing more than one variety.
- Seed source. Use seed from a paddock where viruses and fungal diseases were not detected. A fungal threshold of less than 1% is acceptable. For viruses, a threshold of <0.1% seed infection is necessary in high risk areas, and <0.5% seed infection for low risk areas.
- Seed treatment. Treat seed for sowing with a fungicide seed dressing.
- Canopy management. Manipulate variety choice, row spacing, seeding rate and time of sowing to manage crop height, delay time of canopy closure, and prevent lodging to assist in disease management.
- Wider row spacing (23–42 cm) delays canopy closure, and may reduce disease if lodging does not occur. Standing cereal stubbles, help to keep lentils erect.
- Sowing rate. Aim for a plant population of 100–120 plants per square metre.
- Sowing date. Sow within the optimum planting window for your district.
- Hygiene. Take all necessary precautions to prevent the spread of disease.
- Foliar fungicide program. Foliar fungicide applications often commence before canopy closure. Success depends on monitoring, correct disease identification, timeliness of application and using the correct product.
- Harvest management. Early harvest minimises disease infection on seed.
With an integrated approach to disease management every year, growers can minimise risk and maximise lentil profits in southern Australia. Seasonal conditions and poor crop management play a significant role in disease outbreaks.
Botrytis grey mould (BGM), caused by the pathogens Botrytis cinerea and Botrytis fabae, and ascochyta blight, caused by Ascochyta lentis, are the two major diseases affecting the production of lentil in southern Australia.
Ascochyta blight is favoured by prolonged wet, cool conditions, often before flowering. However, it is most damaging if rain during podding results in pod infection and subsequent downgrading of lentil seed quality due to seed staining.
BGM develops quickly in warm (15–25oC), humid conditions (RH over 80%) for 4–5 days, particularly at flowering and after canopy closure. Yield losses due to BGM arise from infection of lower stems and leaves, which will spread and lead to stem and plant death, and the eventual formation of dead patches within crops which produced small seed or no seed at all.
This bulletin focuses on BGM and ascochyta blight management, but consideration should also be given to other diseases, like viruses and sclerotinia. Sclerotinia develops under similar conditions as BGM and control strategies for BGM help to reduce this disease.
-

A BGM susceptible variety (front centre) showing botrytis grey mould compared with the same variety (immediately behind) treated with fungicide and other varieties (left and right) that have resistance to BGM.
-

Ascochyta lesions and leaf necrosis on lentil leaves.
-

Ascochyta staining on lentil seeds.
-

An integrated disease management plan should include a well-planned fungicide program.
-

Virus patches in lentil. Note initial infection area (dead plants) and newer infections nearby.
-

Beet western yellows virus (BWYV) in lentil.
-

Lentils with CMV and AMV. Note the bare ground surrounding the plants.
On–farm hygiene (post harvest, pre sowing)
Seed retained on-farm should be from the ‘cleanest’ paddocks, where no disease was detected.
The BGM and ascochyta blight pathogens can carryover from one season to the next on infected lentil seed, stubble and volunteer plants. Botrytis can also carry over on the stubble of alternate hosts such as faba bean, vetch, chickpea or lathyrus as they may be a potential harbour of the same botrytis species (Botrytis fabae & B. cinerea) that can attack lentils.
Reduce stubble from potential pulse crop hosts where feasible i.e. bury, destroy or burn infected crop residue. Burying stubble can significantly decrease the decomposition time of stubble.
Grazing helps reduce stubble, however, infected stubble can be carried between paddocks by stock. Be aware of grazing restrictions on stubble treated with fungicides.
Infected stubble may also be carried by wind, water or machinery at harvest. Clean all machinery, transport equipment and storage facilities with compressed air before moving to the next paddock.
Control volunteer lentils, faba beans, vetch, lathyrus and chickpeas early to limit the build-up of disease inoculum.
Paddock selection
A break of at least 3 years should be observed between lentil crops.
Do not sow adjacent to lentil stubble, particularly downwind. If possible, aim to separate this year’s lentil crop from last year’s lentil stubble by a distance of at least 250 m.
Reduce disease risk by not sowing adjacent or into faba bean, chickpea, vetch or lathyrus stubble. If this is not possible, manage the lentil crop with a high botrytis risk management strategy.
Be aware that early sown faba bean, vetch or lathyrus crops may be a source of botrytis inoculum into adjacent, later sown, lentil crops. If this is not possible to avoid, manage the lentil crop with a high botrytis risk management strategy.
Where there is a high risk of sclerotinia, avoid planting lentils after canola or other broadleaf crops which act as alternative hosts to this disease.
Avoid paddocks with high soil nitrogen, as this can lead to greater vegetative growth in lentils, predisposing the crop to disease development.
Ensure the maximum plant-back period for all herbicides is adhered to, as herbicide residues may weaken the plant’s resistance to disease.
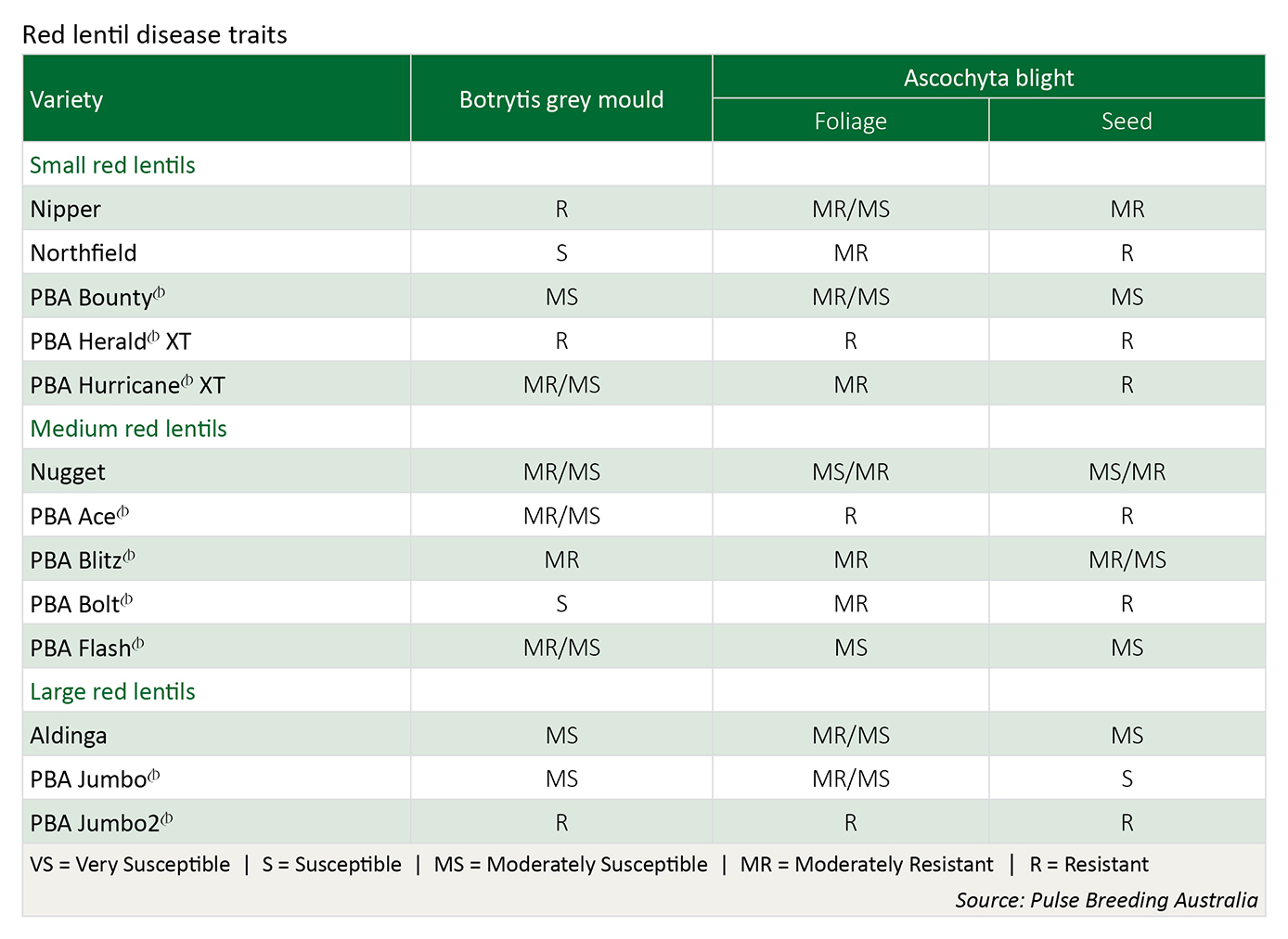
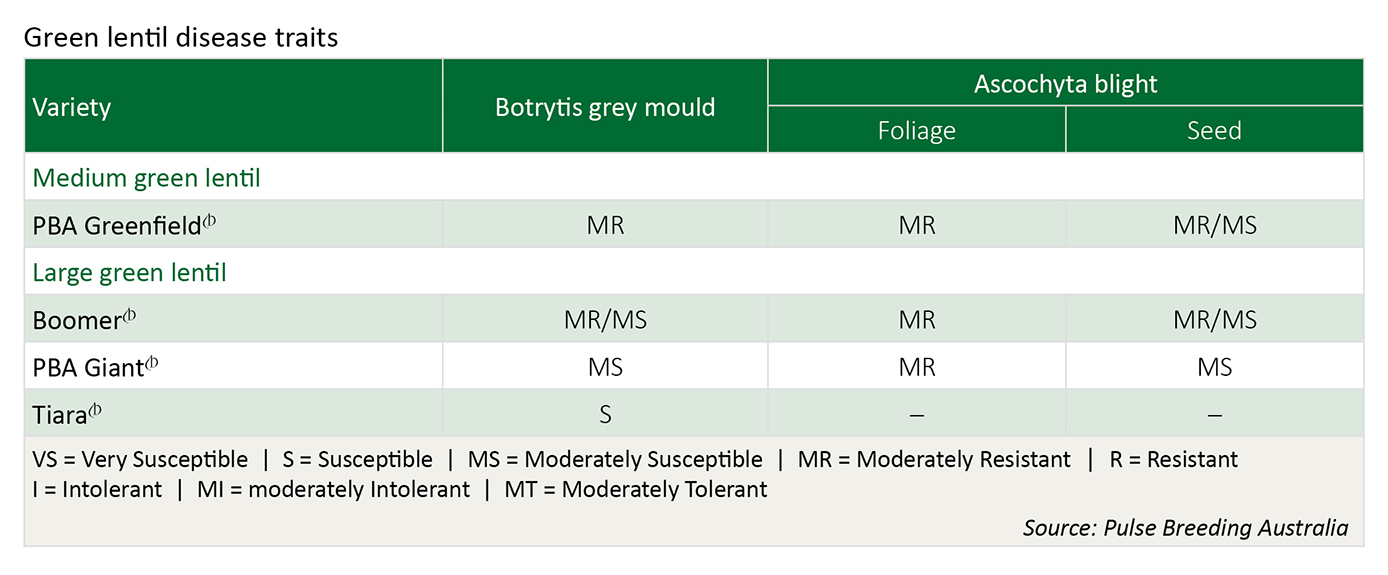
Varieties
Under high disease pressure, all varieties will require fungicide protection to control disease epidemics.
Include a variety that best withstands the main disease risk(s) for your region e.g. PBA Herald-XT, PBA Blitz , PBA Jumbo or Nipper for ascochyta blight resistance; PBA Blitz, PBA herald-XT, Nipper or Boomer for some BGM resistance.
If growing susceptible varieties, spread risks by sowing more than one variety, each with different resistance characteristics. i.e. don’t sow both PBA Flash and Aldinga or both PBA Flash and Northfield if in a BGM prone region.
Quality seed
Seed retained on-farm for sowing should be from the ‘cleanest’ paddocks or section of paddock. Preferably use seed with nil disease infection.
A fungal threshold of less than 1% is acceptable. Definitely avoid using seed with greater than 5% botrytis or 5% ascochyta infection.
Avoid using seed infected with either cucumber mosaic virus (CMV) or alfalfa mosaic virus (AMV). A threshold of <0.1% seed infection is recommended for sowing in high risk areas, and <0.5% seed infection for sowing in low risk areas.
Seed disease testing services are available from:
- SARDI Diagnostic Services Ph 08 8303 9360
- Agrifood Technology Ph 03 9742 0555
- AgWest Laboratories Ph 08 9368 3721.
If seed is more than one year old, frosted, weather damaged or diseased, its germination and vigour may have deteriorated. This may increase its susceptibility to disease attack.
Seed dressing
Treating pulse seed with a fungicide products containing thiram plus thiabendazole reduces the establishment of seed-borne diseases in crops and also protects seed from infection by soil-borne fungi. Some fungicide seed dressings also protect seedlings from external airborne infection for the first few weeks. This is important in reducing the subsequent establishment and spread of disease within crops. Seed treatments provide effective control for a maximum of four to six weeks after sowing, but do not provide absolute control.
- Seed should be properly treated with a fungicide to control seedling root rots and most seed infections, including ascochyta blight and BGM.
- Sowing untreated seed infected by botrytis can result in the development of botrytis seedling blight, which can result in early seedling death and reduce seedling establishment
- Registered seed treatments to use for lentils are: products containing thiram plus thiabendazole (Pickel T, Fairgro or Reaper TT). All are registered in lentils for control of ascochyta and seedling root rots, but will control seedling botrytis as well.
- Seed dressings may have a deleterious effect upon rhizobia so their contact time must be minimised.
- Read the label rhizobia label for compatibilities. Apply seed dressing first and then mix inoculum with seed immediately before sowing.
- Alternatively granular or liquid inoculants are now available and will prevent inoculum coming in contact with fungicide on seed.
Application
It is important for seed treatments to be evenly distributed on seed to ensure each seed gets an effective dose. This is enhanced for flowable seed treatments by dilution with water (refer to the label). Secondary mixing of treated seed through an auger assists to obtain even seed coverage. Correct calibration of the applicator and a consistent seed flow are critical for the recommended rate of seed treatment to be applied.
Some fungicides are toxic to rhizobium, and should not be mixed together before application to seed. Read the labels for compatibilities. Ideally, seed should be treated with fungicide and then, in a separate operation, inoculated with rhizobium just before sowing. Sowing should occur immediately after rhizobium has been applied, particularly in acid soils. Granular or liquid injection of inoculum in-furrow eliminates the contact between seed treatments and rhizobia.
Canopy management
Sowing rate
Aim for a plant population of 100–120 plants per square metre.
Calculate actual seeding rates (kg/ha) based on seed size because seed sizes vary widely with variety and season. Approximations are 50 kg/ha for Nugget or Digger, 60 kg/ha for Aldinga, 40 kg/ha for Nipper or Northfield and 70 kg/ha for Boomer.
Higher seeding rates can increase the risk of BGM due to dense canopy growth.
Sow to minimise overlap, or avoid sowing headlands, as the higher seeding rate can favour the development of a BGM epidemic.
Seeding rates below the minimum recommended plant populations can reduce yield.
Row spacing
Wider row spacing can be part of BGM disease management in lentil by keeping the canopy open and drier for longer, provided lodging does not occur.
Precision sowing lentils between standing cereal stubble rows allows trellising and keeps the lentils more erect. Without stubble trellising, row spacings beyond 23 cm makes the lentil crop susceptible to lodging and increase the risk of disease development. Botrytis can colonise wet cereal straw, but the ability of cereal stubble to act as a multiplier for BGM is still unclear.
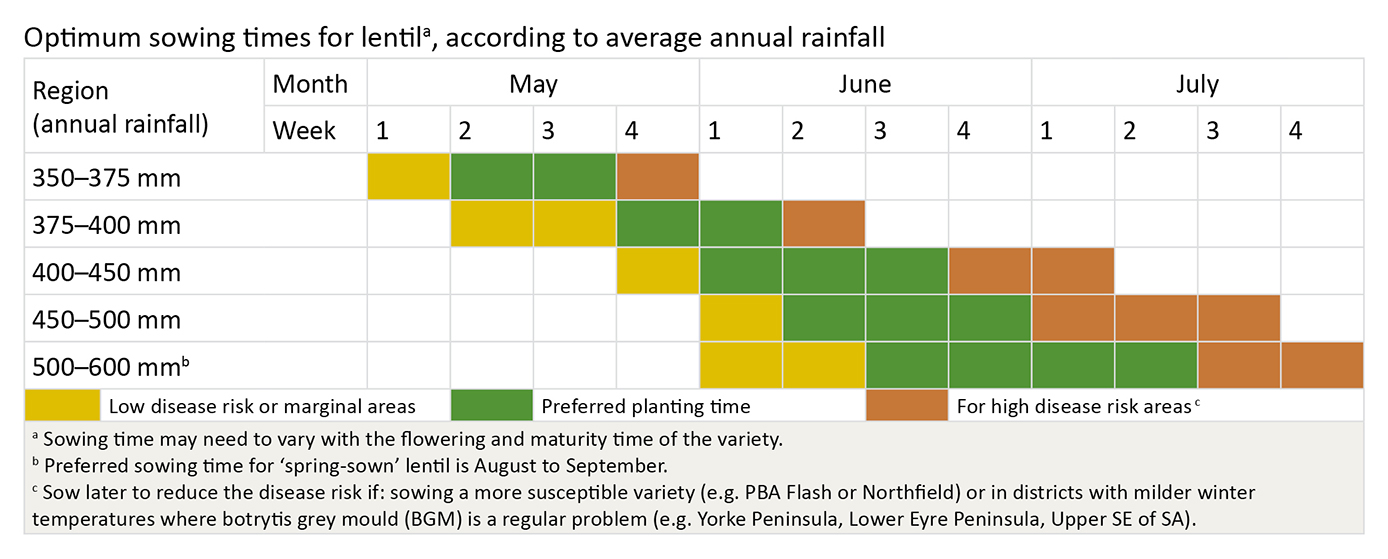
Sowing date
Lentils are quick to emerge, but their early growth is slow, and they can take a long time to completely cover the ground, especially if maximum daily temperatures are below 15oC.
Early sowing results in more vegetative growth and crops prone to lodging, increasing the risk of disease infection and subsequent poor grain quality. Frost risk needs also to be considered.
Later sowings reduce disease risk but can result in lower yields due to the risk of dry conditions, high temperatures at flowering-pod fill and reduced crop height making harvest difficult.
Disease resistant varieties may be sown earlier than other varieties since disease risk is reduced.
Sowing lentils early with wider rows into standing cereal stubble in the belief that it lessens disease risk through delayed canopy closure and less lodging is not proven.
The optimum sowing time for lentils is therefore dependent on location, sowing system used and disease risk:
Early sowing is usually beneficial in medium to low rainfall areas or in areas prone to early, quick finishes to the season. This is provided that weeds, BGM and ascochyta blight are effectively controlled.
Delayed sowing is required in areas where high biomass production occurs in spring, and lodging occurs pre-flowering making effective BGM control more difficult. The ideal is for the lentils to remain standing until flowering has finished.
Spring sowing is desirable in some higher rainfall areas, or areas with a long, late growing season.
In a BGM prone area, sow at the later end of the recommended window for your district and sow varieties like Nipper, Digger or Nugget before varieties like Northfield or Aldinga.
Lentil viruses
When lentil crops in South Australia and Victoria are surveyed, most are found to have cucumber mosaic virus (CMV) present, which can occasionally express as a major problem. In unique cases where lentil crops have experienced heavy losses from viral infection, it has been in association with prolonged, high levels of aphids that arrived early. Combinations of CMV, alfalfa mosaic virus (AMV) and beet western yellow virus (BWYV) are often present in plants tested from these surveys.
The most important factors that predispose pulse crops to severe virus infection are:
- Close proximity to a substantial virus reservoir (eg lucerne, summer weeds, infected lentil seed).
- High summer-autumn rainfall and the subsequent uncontrolled multiplication of aphids on host plants. Early aphid flights to newly emerged crops cause early infection and economic loss as infected plants act as a reservoir for further spread of infection within the crop.
Virus control
Virus risks can be managed by combining a number of different control measures:
- Suppress the virus source within the crop. Sow seed with less than 0.1% seed infection.
- Control volunteer weeds during summer and autumn where lentils are to be sown.
- Decrease aphid landing rates through having stubble cover. Higher seeding rates and narrow row spacing can help too, but can conflict with other fungal disease management needs.
Growers should only consider applying insecticide for virus control if they consider their crops to be at high risk. Insecticides aimed at controlling damage from aphid feeding are normally too late to control virus spread and damage.
See also 'Virus management in pulses'
Fungal diseases
Ascochyta blight risk
A lentil crop is considered to be at risk of ascochyta blight if one or more of the following conditions apply:
- Sown adjacent to old lentil stubble.
- Sown with a susceptible variety.
- Has been sown early.
- History of numerous lentils in the rotation.
- Ascochyta blight is present at commencement of flowering in a susceptible variety.
- If ascochyta blight is present at flowering in a resistant variety during a wet spring.
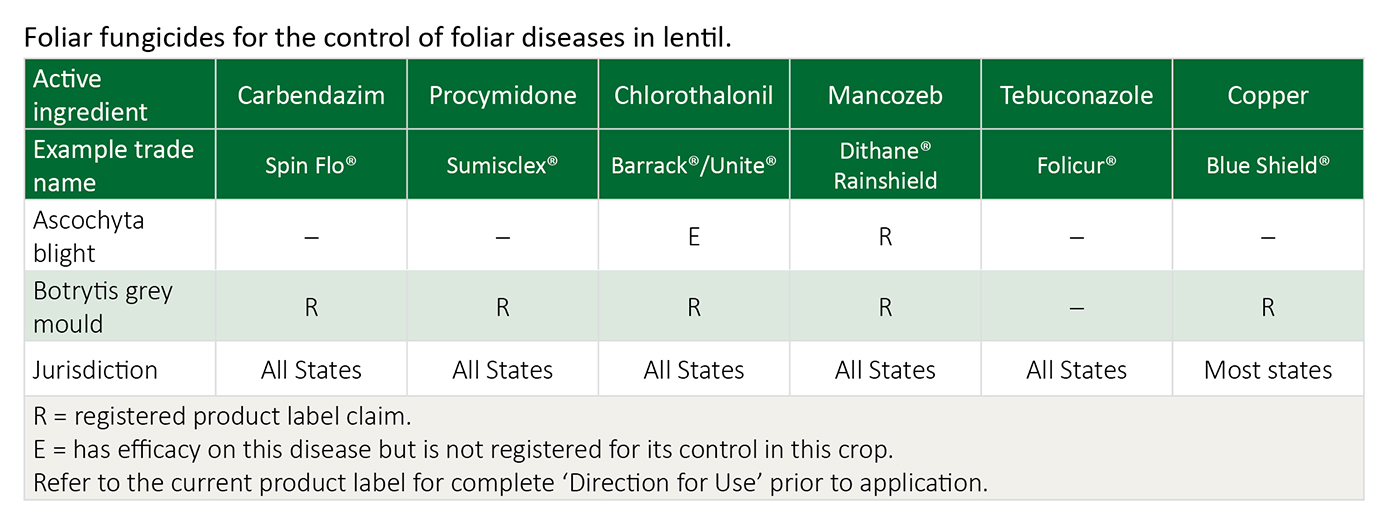
Chlorothalonil is considered a better option than mancozeb when ascochyta persists and its control is most important. Carbendazim and procymidone products give poor protection against ascochyta.
Botrytis grey mould risk
A lentil crop is considered to be at risk of botrytis grey mould if one or more of the following conditions apply:
- Sown adjacent to an old lentil, chickpea, faba bean, vetch or lathyrus stubble.
- Is in a high rainfall area.
- Running total of spring rain is above 20 mm rain (susceptible variety) or above 45mm rain (mod. susceptible variety) and more rain is forecast.
- Forecast night temperatures are above 8–10ºC.
- Was sown early.
- Sown with a susceptible variety.
- Has a plant population of >120 plants per square metre.
- Reached canopy closure by late winter/early spring.
- Has lodged.
If botrytis pressure is high or the disease is spreading in the crop, then carbendazim or procymidone are better options than chlorothalonil or mancozeb. Where mancozeb or chlorothalonil is being used primarily for ascochyta control, it has the added benefit of controlling low levels of botrytis.
Registration regulations limit carbendazim and procymidone to a maximum of two consecutive sprays each. Both are systemic fungicides with single site specificity so the probability of resistance increases with regular use. Alternate the carbendazim or procymidone with a fungicide from a different fungicide group. Procymidone is group 2, carbendazim is group 1. Chlorothalonil is group M5 and mancozeb is group M3.
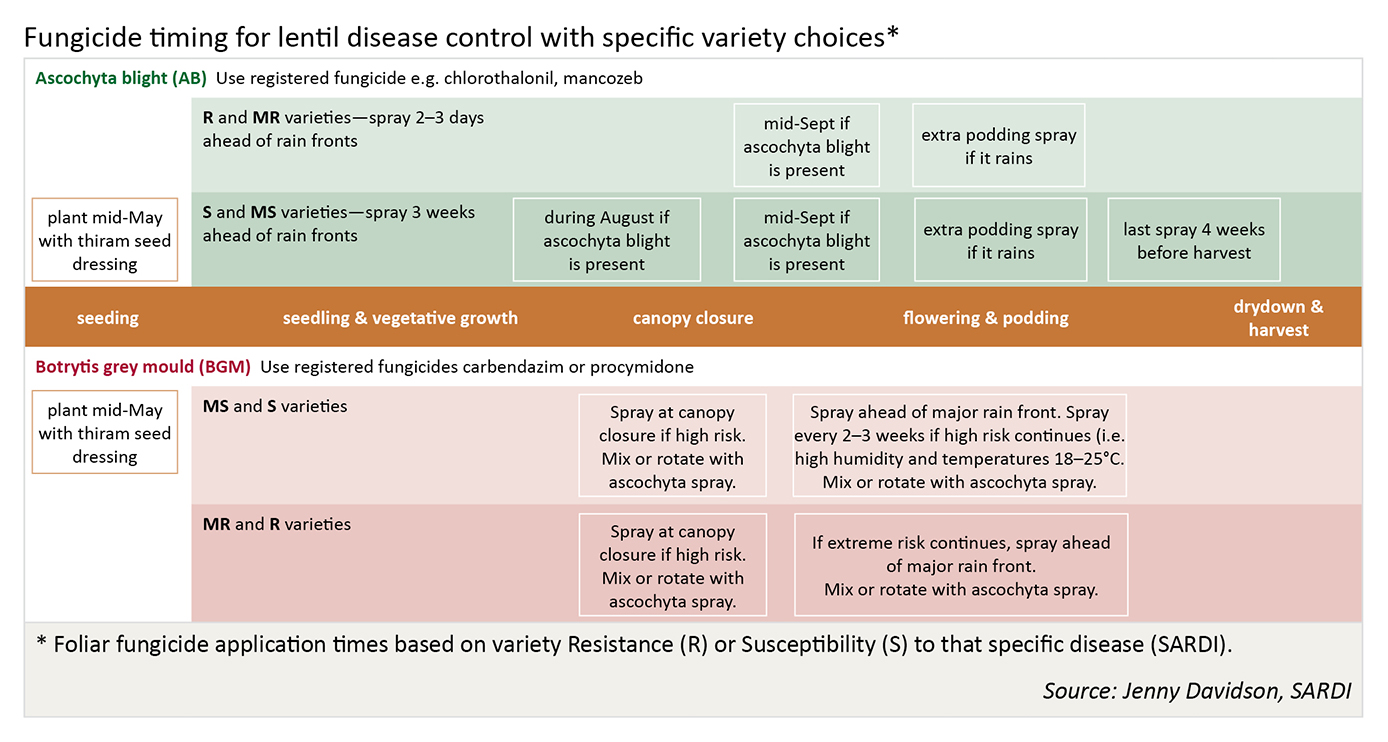
Fungicide spray program for lentils
There are three critical periods for fungicide spraying decisions on lentil crops for disease.
First critical period is at 10–14 weeks after emergence (WAE), shortly prior to canopy closure. Early application of fungicide is critical in restricting the early development and spread of BGM. In susceptible varieties, or in districts prone to BGM epidemics, apply a fungicide, irrespective of BGM being present. An application at this stage is the final chance for spray penetration deep into the crop canopy to protect stems. The presence of ascochyta blight at this stage is unlikely to cause significant yield losses in varieties that have moderate resistance to foliar infection by ascochyta. Varieties like PBA Flash and Tiara that are more susceptible may require ascochyta protection at this early stage.
Second critical period is at mid flowering/early pod fill (14–16 WAE). If either BGM or ascochyta blight is present, or weather is conducive to disease, apply a fungicide. The type of fungicide used will be dependent on the variety. Mixtures of foliar fungicides may be required to give control for both diseases in some susceptible lentil varieties. Continued infection by BGM at this stage will impact on yield by causing stem infection under the canopy and subsequent plant death. Infection of pods by ascochyta at this stage will impact on seed quality and yield if seed is aborted.
Third critical period is at the end of flowering/mid pod fill (16–18 WAE). This is the final growth stage where all pods are formed and protection against ascochyta infection ensures good seed quality is achieved.
If ascochyta blight is present during pod formation and filling, seed staining can occur in many older varieties. PBA Herald-XT, PBA Jumbo and Northfield can be the exception because their seed has resistance to ascochyta infection and subsequent staining. However in a wet spring even these may require a spray during podding to prevent seed staining. Severe disease pressure from ascochyta can result in yield loss in some varieties due to seed abortion, except PBA Blitz, PBA Herald-XT, PBA Jumbo, Nipper and Northfield. Botrytis control may be required though if that disease is severe and weather is conducive to spread.
Requirements, all periods: During the critical periods, monitor crops at least once every week and react by spraying ahead of rain events. Fungicide sprays for BGM are likely to be required depending on variety, rainfall and canopy.
Fungicide application during the critical periods is a standard practice in high disease risk situations, e.g. high rainfall regions, in a wet year or in known disease risk zones. A crop is considered to be at high risk if susceptible varieties are grown, crop rotation is tight, planting is adjacent to lentil stubble, where all preventative management strategies cannot be followed, or any combination of factors put the crop at risk.
Additional fungicide applications may be required, particularly if conditions are conducive to disease.
Take note of grain and grazing withholding periods (WHPs). For crops that are desiccated or windrowed, the WHP is to that date, not actual harvest date.
Effective application
- Control is based on preventing new infections, not curing old infections.
- Stems and lower leaves are not effectively covered by a fungicide applied after canopy closure.
- Spraying before the canopy closes achieves better fungicide penetration and is more effective than trying to halt an epidemic after the canopy closes or the crop has lodged.
- Any new growth after spraying is not protected.
- Spraying ahead of rain prevents fungal infection occurring, rather than waiting until after rain when the disease has already started to spread.
- Spray early in the morning as dew will assist in spread of fungicide product over the plant surface.
- Spraying in light rain is preferable to waiting till after the end of a rain event.
- Depending on the time of year and growth rate of the crop, the period of fungicide protection can vary. In the cooler winter months the period of protection can be for 2–3 weeks when crops growth rates are generally slower. But in warmer spring conditions this period may be reduced to 7–14 days during rapid crop growth and the emergence of unprotected plant growth.
- Use high water rates (preferably 100 L/ha by ground or 30 L/ha by air). Flat fan nozzles with a fine/extra fine droplet spectrum & an operating pressure of 400 kPa is best.
- Surfactants (spreaders) improve initial spray deposits and redistribution (refer to fungicide label).
- Stickers reduce droplet spread over the leaf surface and are not recommended.
Early harvest
Harvest as early as possible to minimise botrytis and ascochyta infection on seed. Disease is usually more severe when crops are harvested late. Harvest losses and downgrading in quality can be substantial if lentil harvest is delayed until moisture content is below 12%.
Marketing problems are created if receival standards of 1% poor colour (due to disease, water staining, frost) are exceeded.
Key contacts
Disclaimer
Information provided in this guide was correct at the time of the date shown below. No responsibility is accepted by Pulse Australia for any commercial outcomes from the use of information contained in this guide.
The information herein has been obtained from sources considered reliable but its accuracy and completeness cannot be guaranteed. No liability or responsibility is accepted for any errors or for any negligence, omissions in the contents, default or lack of care for any loss or damage whatsoever that may arise from actions based on any material contained in this publication.
Readers who act on this information do so at their own risk.
Copyright © 2015 Pulse Australia
All rights reserved. The information provided in the publication may not be reproduced in part or in full, in any form whatsoever, without the prior written consent of Pulse Australia.
Last updated: 20 November 2015

