Australian Pulse Bulletin
Chickpea: Integrated disease management
Summary of strategies
- Variety selection is critical. Ideally grow an ascochyta resistant variety.
- Paddock isolation from chickpea stubble is a high priority (greater than 500 m).
- Paddock history. Aim for a break of at least 4 years between chickpea crops.
- Seed source. Use seed from a paddock where disease was not detected.
- Fungicide seed dressing is effective and should be used, especially in high disease risk situations.
- Sowing date. Do not sow too early, even with an ascochyta resistant variety.
- Sowing depth. If using an ascochyta susceptible variety, sow deeper than normal.
- Sowing rate. Aim for 35–50 plants per square metre, depending on the situation and crop type (kabuli or desi).
- Hygiene. Reduce disease sources and prevent spread of disease.
- Foliar fungicides. Ascochyta resistant varieties still require foliar fungicide at podding. Success is dependent on monitoring, timeliness of spraying and correct fungicide choice. Early detection and correct disease identification are essential.
- Manage aphids and virus. Ground surface cover, healthy plants and crop canopy are important. Control aphids at their source (host) crop.
- Harvest management. Harvest early to minimise disease infection of seed. Crop desiccation enables even earlier harvest.
Knowing the disease
Chickpea crops in Australia are affected by ascochyta blight (Phoma rabiei), botrytis grey mould (Botrytis cinerea), sclerotinia (Sclerotinia spp), phoma blight and other seedling root diseases such as Pythium spp, Fusarium spp and Rhizoctonia spp. Some viruses and pratylenchus root lesion nematode (Pratylenchus neglectus, P. thornei) can affect chickpea. Disease management of chickpea in all regions should primarily focus on ascochyta blight. Phytophthora root rot is a most damaging disease in northern NSW and Queensland, but is not considered a problem in southern areas.
For specific information related to identifying and managing each crop, refer to the following resources:
- Ascochyta blight management
- Botrytis grey mould
- Phytophthora root rot
- Sclerotinia
- Root lesion nematodes
- Viruses
Disease carry-over and on–farm hygiene
Post-harvest to pre-sowing precautions include:
- Protect seed crops with fungicides at podding. Seed retained on-farm should be as free of ascochyta inoculum as possible.
- Control volunteer chickpeas pre seeding to limit build-up of disease inoculum for the new crop.Ascochyta and botrytis grey mould carry from one season to the next on infected chickpea stubble, infected seed or volunteer plants.
- Particularly with ascochyta susceptible varieties, undertake a program of stubble reduction (e.g. chop, bury, destroy, graze or burn infected crop residue) where this will not create an erosion risk.
- Clean all machinery, transport equipment and storage silos with compressed air before moving to the next paddock. Infected stubble may also be carried by wind, water and machinery at harvest.
Paddock selection
Avoid planting into or in close proximity (particularly downwind) to chickpea stubble and volunteer chickpea plants. Aim to maintain a distance of at least 500 m between new plantings and stubble from previous years.
Be aware that self-sown chickpeas or early sown chickpea crops may be a source of ascochyta inoculum that can spread into adjacent, later sown chickpea crops.
A break of at least 4 years between chickpea crops is usually required to minimise the amount of inoculum present in the soil. In paddocks that grew faba bean, chickpea, vetch, lathyrus, field pea, medic or clover pastures two years previously, there is a slight risk of phoma (Phoma medicaginis var pinodella), sclerotinia or botrytis grey mould attacking chickpea.
There is a greater risk of botrytis grey mould in chickpea if sown downwind from a crop or infected stubble of lentils or faba beans.
Observe the maximum plant-back period for sulphonylurea herbicides (e.g. Glean®, Logran®), Lontrel®, triazines and imidazolinones (e.g. Spinnaker®). Herbicide residues may increase susceptibility to disease (including viruses).
Crop stress can occur in paddocks with low soil fertility or nutrient status, predisposing the chickpea plants to disease attack.
Varieties
Yield and marketability, along with disease resistance, are the major factors to consider in variety choice (see table below). Resistant varieties are not immune to that disease, and may suffer some production losses or grain quality damage under high disease pressure. All varieties are susceptible to ascochyta blight during podding.
More detailed information: [link to disease rating section of the Chickpea BMP guide/s]
Seed
Select seed of the highest possible purity, germination and disease free status. For ascochyta susceptible varieties, use seed with nil disease infection because ascochyta epidemics can be initiated by very low levels of seed infection. Ascochyta infected seed will often fail to emerge, but can act as a source of disease when transported to new districts.
Botrytis grey mould can occur on seed and significantly affect establishment if not treated with a fungicide seed dressing.
Seed retained on-farm should be from the ‘cleanest’ paddock or section. Select the area early, apply fungicides at podding and harvest the seed crop ahead of other chickpea paddocks to prevent contamination from potentially diseased chickpea crops.
If seed is more than one year old, frosted, weather damaged or diseased, its germination and vigour may have deteriorated. This may increase the crop’s susceptibility to disease attack.
Re-test seed for germination percentage before sowing if retained in silos for more than one year.
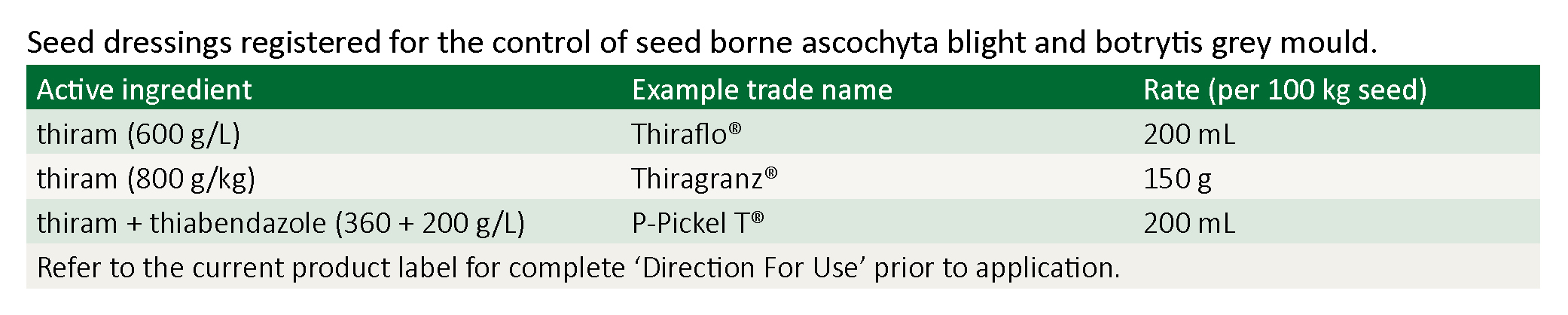
Seed dressing
No chickpea variety is resistant to seed infection by ascochyta or botrytis grey mould. Treat all kabuli types and all ascochyta susceptible desi varieties with a seed dressing. All ascochyta resistant varieties, desi or kabuli, will benefit from a seed dressing to protect against botrytis and other seedling rots (see table below). Seed dressings may have a deleterious effect upon rhizobia, particularly under acid soil conditions, so minimise the contact time between these seed treatments. Check the inoculum label. Either apply seed dressing first, then separately mix the inoculum and apply it to the seed immediately before sowing, or consider using granular or liquid injection rhizobia inoculums.
Sowing date
Sow within optimum sowing window for the region.
With ascochyta resistant varieties, sow at traditional sowing dates. Delayed sowing for ascochyta risk reduction is not necessary in those varieties.
With ascochyta susceptible varieties, delayed sowing is an important strategy for ascochyta management. It reduces the duration of exposure of chickpea seedlings to ascochyta spores. It will not help though if self-sown chickpeas are nearby. Be aware that delayed sowings can result in lower yields due to increased risk of a dry finish and high temperatures during podding.
In all varieties, sowing too early can lead to poor early pod set or seed fill if flowering in a colder period (less than 15°C mean daily temperature).
Early sowing can produce increased vegetative growth and may increase lodging, which will increase the risk of botrytis grey mould incidence, particularly in the larger plant types.
In a disease prone area or higher risk situation, sow at the later end of the recommended optimum for the district.
More detailed information: [insert link to sowing date section of Chickpea BMP guide/s]
Sowing rate
Aim for 35–50 plants per square metre, depending on the situation and crop type (kabuli or desi). Higher seeding rates lead to greater canopy vigour, increased lodging and higher humidity, and under ideal growing conditions can increase the risk of botrytis grey mould.
Avoid double sowing headlands. A denser crop can be more prone to disease establishment and lodging.
Seeding at rates below the minimum recommended plant population will have minimal impact on fungal disease incidence, but reduce potential yield and increase harvest losses. A lower seeding rate may increase aphid landing, hence virus infection.
More detailed information: [insert link to sowing rate section of Chickpea BMP guide/s]
Row spacing
Wider row spacing does not reduce ascochyta incidence in chickpea, but could reduce the occurrence of botrytis grey mould. A wider configuration may also allow better fungicide penetration and cover.
Use of wider row spacing with chickpea sown into standing stubble is part of an overall farming system targeting water use efficiency, weed control and harvestability rather than specifically for disease control.
More detailed information: Wide row and stubble retention in pulses
Disease monitoring and control
Disease impact is greatly reduced when a fully integrated disease management program is initiated before seeding and maintained through the growing season. A crop is considered to be at high risk if a susceptible variety is grown, crop rotation is tight, it is sown adjacent to chickpea stubble, sown early or where all integrated management strategies cannot be followed.
Potentially critical periods for disease development in chickpea are during the early vegetative stages (for ascochyta susceptible varieties), flowering and seed fill. The decision to spray or not will depend on the variety resistance rating and the disease risk for the individual crop. Start disease monitoring 6–8 weeks after emergence of a susceptible crop since regular fungicide applications will be necessary in these varieties.
Ascochyta blight (Phoma rabiei)
A seed treatment containing thiram (Thiragranz® or Thiraflo ST®) or thiram plus thiabendazole (P-Pickel T®) is essential to control seed-borne infection.
Chlorothalonil is the more effective foliar fungicide for ascochyta, and only Unite® 720 and Barrack® 720 are registered for this use. Mancozeb and metiram can also provide some protection from ascochyta if timeliness of application is observed. For example, mancozeb might give protection for a fortnight compared to a possible 21 days protection following chlorothalonil application.
Efficacy differences between products can arise if ascochyta is present. Protective fungicide sprays should be applied 1–3 days ahead of rainfall to prevent infection spreading during the rainy period. No new ascochyta infection will occur during dry periods, so fungicide application can be held off until rain (>5 mm) is forecast. Do not wait until after the rain.
With ascochyta resistant varieties like PBA Slasher, Genesis 090 or Genesis 079, spraying for ascochyta before the podding stage is unlikely to be needed, especially if no ascochyta lesions are present. A single application of chlorothalonil at podding may be sufficient for grain protection in these more resistant cultivars. A repeat application should only be necessary in severe and extended disease pressure situations (rainfall during spring combined with presence of disease in the crop).
More detailed information: Managing ascochyta blight
Botrytis grey mould (Botrytis cinerea)
If infected seeds are sown, botrytis seedling blight can reduce plant establishment. Seed treatment containing thiram (Thiragranz®, Thiraflo ST®, Thiraflo FF® or Thiram®) or thiram plus thiabendazole (P-Pickel T®, Fairgro® or Reaper®TT) will control seed-borne infection.
Warm, humid conditions under a dense crop canopy in spring are ideal for the spread of botrytis grey mould. Carbendazim or procymidone are most effective against a botrytis grey mould (BGM) epidemic. Use of chlorothalonil, mancozeb or metiram as a foliar spray for control of ascochyta has the added benefit of providing some protection from and control of BGM. Applications targeted at ascochyta during podding might however be too late to protect flowers for BGM infection.
More detailed information: Managing botrytis grey mould
Phytophthora root rot (Phytophthora megasperma)
Phytophthora is a soil and water-borne disease that can establish permanently in some paddocks. It is generally not a problem in southern growing regions.
Phytophthora damage is greatest in seasons with above average rainfall. However, only a single saturating rain event is needed for infection to occur.
Once a plant or crop is infected with phytophthora, there is nothing a grower can do. Unlike ascochyta and botrytis, crops cannot be sprayed to prevent or treat phytophthora. Thus phytophthora can only be managed through pre-sowing decisions.
The most effective control strategy is to not sow chickpeas in high-risk paddocks. High risk paddocks have a history of:
- Phytophthora noted in previous chickpea or lucerne crops.
- Lucerne or annual or perennial medics production.
- Waterlogging or prone to flooding.
If considerations other than phytophthora warrant sowing in a high-risk paddock, choose PBA HatTrick or Yorker, which have a moderate level of resistance to the disease.
More detailed information: Managing phytophthora root rot
Root lesion nematodes (RLN) (Pratylenchus thornei, P. neglectus)
Root lesion nematodes cause poor plant growth in situations that otherwise appear favourable. They attack cereals and pulses and are thus a threat to the whole farming system.
The nematodes feed and multiply on and in the roots of chickpea plants and, when in sufficient numbers, reduce growth and yield. Chickpea varieties differ in their resistance and tolerance to RLN but are generally considered more susceptible (allowing nematodes to multiply) than field pea, faba bean and lupin, but less so than wheat.
Reduce the risk of loses from RLN by not sowing chickpea in paddocks that had susceptible or intolerant cereal varieties in recent years.
More detailed information: Managing root lesion nematodes
Sclerotinia base rot (Sclerotinia sclerotiorum, S. minor) and Sclerotinia aerial blight (S. sclerotiorum)
There are two species of Sclerotinia that attack chickpeas and they can be distinguished by the size of their sclerotes (survival structures).
S. sclerotiorum is the more prevalent species in cooler wetter regions whilst S. minor is more common in warmer drier environments. Both species cause basal stem rot when their sclerotia germinate in soil and infect the base of the plant.
Both species of sclerotinia have wide host ranges including many broadleaf weeds and crops such as canola, faba bean and sunflower. However, cotton and cereals do not host either species.
Reduce the risk of losses from sclerotinia by sowing seed free of sclerotia and by not sowing chickpea in paddocks that have had alternative host crops in the past 10 years because the resting structures (sclerotes) can survive that long. Maintaining a full 10 year break is generally impracticable, so a value judgement will usually be required taking into account the various risk factors.
No fungicides are registered or under permit for sclerotinia in chickpea and it’s unlikely that any would be effective against the basal stem rot phase of the disease.
More detailed information: Identifying sclerotinia
Viruses (several species)
Chickpea crops in South Australia and Victoria are prone to Beet western yellows virus (BWYV), which on occasions has been devastating. BWYV is not seed-borne. Cucumber mosaic virus (CMV) is also present and can occasionally express as a major problem. CMV is seed-borne. Where chickpea crops have experienced heavy losses from viral infection it has mostly been associated with prolonged, high levels of aphids that arrived early from nearby. There is often an association between virus susceptibility and thin plant populations, bare ground or plants that were stressed by factors such as herbicide damage, transient wet conditions, phoma and / or slow growth from cold conditions. Lack of virus infection under cold conditions is most likely associated with low aphid numbers.
Important factors that predispose pulse crops to severe virus infection are:
- Close proximity to a substantial build up of aphids or a nearby reservoir of aphids (e.g. in lentil, canola, lucerne, summer weeds).
- High summer-autumn rainfall and the subsequent uncontrolled multiplication of aphids on host species nearby. Aphids do not colonise chickpea, but fly in and out, spreading virus as they probe plant tissue to feed.
- Infected chickpea seed.
- Early aphid flights to newly emerged crops cause early infection and economic loss as infected plants act as a reservoir for further spread of infection within the crop.
- Thin plant populations with areas of bare ground.
Virus control
To reduce the risk of virus infection, combining a number of different control measures from this list:
- Suppress the virus source within the crop by sowing seed with less than 0.1% seed infection.
- During summer and autumn, control volunteer weeds in and nearby where chickpea is to be sown.
- Ensure chickpea plants are less attractive to aphids by minimising seedling diseases, herbicide damage or poor nutrition.
- Decrease aphid landing rates through having stubble cover and / or a dense early canopy. Note that high seeding rates and narrow row spacing to provide early canopy closure assists in aphid control, but may conflict with other fungal disease and crop moisture management systems.
Note that seed treatment of imidacloprid is not registered in chickpea for early aphid protection.
Control aphids in nearby host crops to prevent an aphid population build up before they enter the chickpea crop. Only apply insecticide for virus control in the chickpea crop if it is considered to be at high risk. Insecticide aimed at controlling damage from aphid feeding are normally applied too late to control virus spread.
More detailed information: Virus control in pulses
Foliar fungicide application guide
Consider the variety grown, potential crop yield, rainfall zone and disease risk when deciding on fungicide use.
Treat chickpea seed, particularly kabuli varieties, with a seed dressing to protect against seedling root rots as well as to provide early protection against ascochyta blight. Where there is a high risk of ascochyta (e.g. adjacent chickpea stubbles, early sown, close rotations), treat seed for ascochyta protection, irrespective of variety.
With ascochyta resistant varieties (e.g. PBA Slasher, Genesis 090, Genesis 509), only consider applying an early foliar fungicide for ascochyta if the disease is present. A foliar fungicide applied ahead of rain during podding will likely be required to protect grain quality. Varieties with resistance to ascochyta require fewer and later fungicide applications for ascochyta control. This reduction in fungicide use may expose the crop to a higher risk of botrytis grey mould infection.
With varieties that are intermediate in ascochyta resistance (e.g. Kalkee, Genesis 114, Almaz, Genesis 836), three to four strategic foliar fungicide applications for ascochyta control will be necessary in many districts. Apply a fungicide early, before the disease is detected pre-flowering. Strategic applications will likely be needed at the commencement of flowering, early podding and late podding. Timing of the protective application is critical, as control is less effective if the fungicide is applied after the disease has taken hold.
With ascochyta susceptible varieties (e.g. Howzat, Kaniva, Sonali), regular foliar fungicide applications for ascochyta control will be necessary in all districts. Apply a fungicide spray before the disease is detected, from emergence through flowering until 4 weeks before maturity. Starting early with protective applications is critical. Control is often ineffective if fungicide is applied after the disease has taken hold.

A fungicide program needs to account for:
Disease risk categories, based on:
- varietal susceptibility or resistance
- source of seed and treatment of seed
- planting proximity to chickpea crops in the previous season
- level of ascochyta inoculum present from crop residue or volunteer plants
- climatic conditions in relation to disease infection.
Registration status: The product must be registered or have a permit for the disease and use.
Withholding period: All products and timings used in the fungicide program must meet Australian withholding periods and export slaughter intervals.
Fungicide resistance management: Adhere to the maximum number of sprays of a product to minimise the risk of fungicide resistance developing.
Mode of action: To further reduce the chance of fungicide resistance development and to improve efficacy, use products with a range of mode of actions. Fungicides are also recommended at times of the disease life cycle where they will be most effective according to their mode of action.
Cost effectiveness.
Early harvest
Harvest at maturity to minimise ascochyta seed infection and potential downgrading. Seed damage from ascochyta is usually more severe when crops are harvested late. Moisture content allowable on delivery is 14%. Harvest losses, seed splitting and downgrading in quality can be substantial if chickpea is harvested at below 12% moisture.
Chickpea crop desiccation assists in early harvest. Care is needed in desiccating because of the late maturity of chickpea. Do not croptop or prematurely desiccate as it can affect grain quality, particularly kernel or seed coat colour.
More detailed information: Desiccation and croptopping of pulses
Early harvest will give the best chance of achieving number 1 grade chickpea receival standard, in which there is a maximum of 1% poor colour (due to disease, water staining, weathering or frost).
Useful resources
- Chickpea disease management factsheets—northern and southern regions (GRDC)
- Chickpea disorders: Ute guide (GRDC saleable publication)
Contributions from Jenny Davidson and Larn McMurray (SARDI), Kevin Moore, Kristy Hobson, Kurt Lindbeck and Leigh Jenkins (DPI NSW), Jason Brand (DPI Vic.), Mal Ryley (DAF), Gordon Cumming and Wayne Hawthorne (Pulse Australia) are acknowledged.
Key contacts
Disclaimer
Information provided in this guide was correct at the time of the date shown below. No responsibility is accepted by Pulse Australia for any commercial outcomes from the use of information contained in this guide.
The information herein has been obtained from sources considered reliable but its accuracy and completeness cannot be guaranteed. No liability or responsibility is accepted for any errors or for any negligence, omissions in the contents, default or lack of care for any loss or damage whatsoever that may arise from actions based on any material contained in this publication.
Readers who act on this information do so at their own risk.
Copyright © 2015 Pulse Australia
All rights reserved. The information provided in the publication may not be reproduced in part or in full, in any form whatsoever, without the prior written consent of Pulse Australia.
Last updated: 30 December 2015
